BRIEF: The Advisory Committee on Immunization Practices [ACIP] and Its Exposure, Culpability and Liability for Adverse “Vaccine” Injury and Death
A non-journalistic and technical brief for litigation to identify the relevant statutes, agencies, departments and individuals that bear the duty to provide advice and guidance on U.S. vaccination
Date: 05 Nov 22
The purpose of this non-journalistic and technical brief is to:
Identify the relevant statutes, agencies, departments and individuals that bear the duty to provide advice and guidance to the Director of the CDC regarding use of vaccines and related agents for effective control of vaccine-preventable diseases in the civilian population of the United States
Identify vectors for litigation against those agencies, departments and individuals
To assist and lend assistance to an individuals, families and their attorneys pursuing litigation for adverse reactions up to death caused by mRNA injections for COVID-19
APPLICABLE STATUTES
The applicable statutes in this brief include:
Section 222 of the Public Health Service Act (42 U.S.C. §2l7a)
Federal Advisory Committee Act, as amended, 5 U.S.C. App 2)
Subsection 1928(c)(2)(B)(i)
Subsection 1928(e) of the Social Security Act (42 U.S.C. § 1396s(c)(2)(B)(i)
Subsection 1396s(e))
Subsection 2713(a)(2) of the Public Health Service Act (42 U.S.C. § 300gg-13(a)(2))
Section 311 Public Health Service Act, [42 U.S.C. §243 and 42 U.S.C. §247b]
Section 317 Public Health Service Act, [42 U.S.C. §243 and 42 U.S.C. §247b]
Section 1928 of the Social Security Act
Affordable Care Act (Section 2713 of the Public Health Service Act)
Government in the Sunshine Act (5 U.S.C. § 552b(c)
Section 10(d) of the Federal Advisory Committee Act
Freedom of Information Act, 5 U.S.C. §552
IDENTIFIED AGENCIES & DEPARTMENTS
The identified agencies and departments directly involved in or associated with this brief include:
Advisory Committee on Immunization Practices [ACIP]
The Centers for Disease Control [CDC]
Human and Health Services [HHS]
General Services Administration [GSA]
IDENTIFIED INDIVIDUALS
The identified individuals in this brief include:
Director of the CDC: Robert Redfield [former], Rochelle Walensky [current]
Secretary of HHS: Alex Azar [former], Xavier Becerra [current]
ACIP Committee Members:
LEE, Grace M., MD, MPH [Chair]
Associate Chief Medical Officer for Practice Innovation
Lucile Packard Children’s Hospital
Professor of Pediatrics, Stanford University School of Medicine
Stanford, CA
Term: 8/4/2021 – 6/30/2023
WHARTON, Melinda, MD, MPH [Executive Secretary]
Associate Director for Vaccine Policy
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
Atlanta, GA
BAHTA, Lynn, RN, MPH, CPH
Immunization Program Clinical Consultant
Infectious Disease, Epidemiology, Prevention & Control Division
Minnesota Department of Health
Saint Paul, Minnesota
Term: 7/1/2019 – 6/30/2023
BELL, Beth P., MD, MPH
Clinical Professor
Department of Global Health, School of Public Health
University of Washington
Seattle, WA
Term: 7/1/2019 – 6/30/2023
BROOKS, Oliver, MD, FAAP
Chief Medical Officer
Watts HealthCare Corporation
Los Angeles, CA
Past President, National Medical Association
Term: 7/26/2021 – 6/30/2025
CHEN, Wilbur H., MD, MS, FACP, FIDSA
Professor of Medicine
Center for Vaccine Development and Global Health
University of Maryland School of Medicine
Baltimore, MD
Term: 12/23/2020 – 6/30/2024
CINEAS, Sybil, MD, FAAP, FACP
Associate Professor of Medicine, Pediatrics, and Medical Science (Clinical)
The Warren Alpert Medical School of Brown University
Associate Program Director
Brown Combined Residency in Internal Medicine and Pediatrics
Providence, RI
Term: 9/28/2021 – 6/30/2025
DALEY, Matthew F., MD
Senior Investigator
Institute for Health Research
Kaiser Permanente Colorado
Aurora, CO
Term: 1/4/2021 – 6/30/2024
KOTTON, Camille Nelson, MD, FIDSA, FAST
Clinical Director, Transplant and Immunocompromised Host Infectious Diseases
Infectious Diseases Division, Massachusetts General Hospital
Associate Professor of Medicine, Harvard Medical School
Boston, MA
Term: 12/23/2020 – 6/30/2024
LOEHR, Jamie, MD, FAAFP
Owner, Cayuga Family Medicine
Ithaca, New York
Term: 7/26/2021 – 6/30/2025
LONG, Sarah S., MD
Professor of Pediatrics
Drexel University College of Medicine
Section of Infectious Diseases
St. Christopher’s Hospital for Children
Philadelphia, Pennsylvania
Term: 12/24/2020 – 6/30/2024
MCNALLY, Veronica V., JD
President and CEO
Franny Strong Foundation
West Bloomfield, Michigan
Term: 10/31/2018 – 6/30/2023
POEHLING, Katherine A., MD, MPH
Professor of Pediatrics and Epidemiology and Prevention
Director, Pediatric Population Health
Department of Pediatrics
Wake Forest School of Medicine
Winston-Salem, NC
Term: 7/1/2019 – 6/30/2023
SÁNCHEZ, Pablo J., M.D.
Professor of Pediatrics
The Ohio State University – Nationwide Children’s Hospital
Divisions of Neonatal-Perinatal Medicine and Pediatric Infectious Diseases
Director, Clinical & Translational Research (Neonatology)
Center for Perinatal Research
The Research Institute at Nationwide Children’s Hospital
Columbus, Ohio
Term: 7/1/2019 – 6/30/2023
SHAH, Nirav D., MD, JD
Director
Maine Center for Disease Control and Prevention
Augusta, ME
Term: 9/26/2022 – 6/30/2026
TALBOT, Helen Keipp, MD
Associate Professor of Medicine
Vanderbilt University
Nashville, TN
Term: 10/29/2018 – 6/30/2023Ex-officio members:
Centers for Medicare and Medicaid Services (CMS)
HANCE, Mary BethSenior Policy Advisor
Division of Quality, Evaluations and Health Outcomes
Children and Adults Health Programs Group
Center for Medicaid, CHIP and Survey & Certification
Centers for Medicare and Medicaid Services
Baltimore, MD
Food and Drug Administration (FDA)
FINK, Doran, MD, Ph
Deputy Director, Clinical, Division of Vaccines and Related Products Applications
Office of Vaccines Research and Review
Center for Biologics Evaluation and Research
Food and Drug Administration
Silver Spring, MD
Health Resources and Services Administration (HRSA)
GRIMES, Reed, MD, MPH
CDR, USPHS
Director, Division of Injury Compensation Programs
Health Systems Bureau
Health Resources and Services Administration
Rockville, MD
Indian Health Service (IHS)
CLARK, Matthew, MD, FAAP, FACP
Physician
Chair, IHS National Pharmacy & Therapeutics Committee
Durango, CO
Office of Infectious Disease and HIV/AIDS Policy (OIDP)
KIM, David, MD, MA
Director, Division of Vaccines, OIDP
Office of the Assistant Secretary for Health
Department of Health and Human Services
Washington, DC
National Institutes of Health (NIH)
BEIGEL, John, M.D.
Associate Director for Clinical Research
Division of Microbiology and Infectious Diseases
National Institute of Allergy and Infectious Diseases (NIAID)
Bethesda, MDLiaison Representatives:
American Academy of Family Physicians (AAFP)
ROCKWELL, Pamela G., D.O.
Professor, Department of Family Medicine,
University of Michigan Medical School
Medical Director, Dominos Farms Family Medicine
Ann Arbor, MI
American Academy of Pediatrics (AAP)
O’LEARY, Sean, MD, MPH
Professor of Pediatrics
Pediatric Infectious Diseases
General Academic Pediatrics
Children’s Hospital Colorado
University of Colorado School of Medicine
American Academy of Pediatrics (AAP) Red Book Editor
KIMBERLIN, David, MD
Professor of Pediatrics
Division of Pediatric Infectious Diseases
The University of Alabama at Birmingham School of Medicine
Birmingham, AL
American Academy of Physician Associates (AAPA)
LÉGER, Marie-Michèle, MPH, PA-C
Senior Director, Clinical and Health Affairs
American Academy of Physician Assistants
Alexandria, VA
American College Health Association (ACHA)
CHAI, Thevy S., MD
Director of Medical Services
Campus Health Services
University of North Carolina at Chapel Hill
Chapel Hill, NC
American College Health Association (ACHA) (alternate)
MCMULLEN, Sharon, RN, MPH, FACHA
Assistant Vice President of Student & Campus Life for Health and Wellbeing
Cornell Health
Ithaca, NY
American College of Nurse Midwives (ACNM)
HAYES, Carol E., CNM, MN, MPH, FACNM
Adjunct Professor
Georgia State University School of Nursing
Atlanta, GA
American College of Nurse Midwives (ACNM) (alternate)
MEHARRY, Pamela M., PHD, CNM
Midwifery Educator, Human Resources for Health
In partnership with University of Rwanda and University of Illinois, Chicago
American College of Obstetricians and Gynecologists (ACOG)
ECKERT, Linda O., MD, FACOG
Professor, Department of Obstetrics & Gynecology
Adjunct Professor, Department of Global Health
University of Washington
Seattle, WA
American College of Physicians (ACP)
GOLDMAN, Jason M. MD, FACP
Affiliate Assistant Professor of Clinical Biomedical Science, Florida Atlantic
University, Boca Raton, Florida
Private Practice
Coral Springs, FL
American Geriatrics Society (AGS)
SCHMADER, Kenneth, MD
Professor of Medicine-Geriatrics
Geriatrics Division Chief
Duke University and Durham VA Medical Centers
Durham, NC
America’s Health Insurance Plans (AHIP)
GLUCKMAN, Robert A., MD, MACP
Chief Medical Officer, Providence Health Plans
Beaverton, OR
American Immunization Registry Association (AIRA)
COYLE, Rebecca, MSEd
Executive Director, AIRA
Washington, DC
American Medical Association (AMA)
FRYHOFER, Sandra Adamson, MD
Adjunct Associate Professor of Medicine
Emory University School of Medicine
Atlanta, GA
American Nurses Association (ANA)
RITTLE, Charles (Chad), DNP, MPH, RN
Associate Professor, Nursing
Chatham University, School of Health Sciences
Pittsburgh, PA
American Osteopathic Association (AOA)
GROGG, Stanley E., D
Associate Dean/Professor of Pediatrics
Oklahoma State University-Center for Health Sciences
Tulsa, OK
American Pharmacists Association (APhA)
HOGUE, Michael D., PharmD, FAPhA, FNAP
Dean and Professor of Loma Linda University School of Pharmacy
Director, Center for Interprofessional Education & Practice
Loma Linda, CA
Association of Immunization Managers (AIM)
HOWELL, Molly, MPH
Immunization Program Manager
North Dakota Department of Health
Bismarck, ND
Association for Prevention Teaching and Research (APTR)
ZIMMERMAN, Richard, MD, MPH
Professor
University of Pittsburgh School of Medicine
Department of Family Medicine and Clinical Epidemiology
Pittsburgh, PA
Association of State and Territorial Health Officials (ASTHO)
TBA
Biotechnology Industry Organization (BIO)
ARTHUR, Phyllis A., MBA
Senior Director, Vaccines, Immunotherapeutics and Diagnostics Policy
Washington, DC
Council of State and Territorial Epidemiologists (CSTE)
HAHN, Christine, MD
State Epidemiologist
Office of Epidemiology, Food Protection and Immunization
Idaho Department of Health and Welfare
Boise, ID
Council of State and Territorial Epidemiologists (CSTE) (alternate)
LETT, Susan, MD, MPH
Medical Director, Immunization Program
Division of Epidemiology and Immunization
Massachusetts Department of Public Health
Boston, MA
Canadian National Advisory Committee on Immunization (NACI)
DEEKS, Shelley, MD, MHSc, FRCPC, FAFPHM
Deputy Chief Medical Officer of Health, Department of Health and Wellness, Nova Scotia
Associate Professor, Dalla Lana School of Public Health, University of Toronto
Chair, National Advisory Committee on Immunization
Halifax, Nova Scotia
Infectious Diseases Society of America (IDSA)
DUCHIN, Jeffrey, MD
Health Officer, Public Health – Seattle and King County
Professor in Medicine, Division of Allergy and Infectious Diseases
University of Washington School of Medicine and School of Public Health
Seattle, WA
Infectious Diseases Society of America (IDSA) (alternate)
MCAULEY, James B., DTM&H, MD, MPH
Clinical Director, Whiteriver Indian Hospital
Whiteriver, AZ
International Society for Travel Medicine (ISTM)
BARNETT, Elizabeth D, MD
Professor of Pediatrics
Boston University School of Medicine
Boston, MA
National Association of County and City Health Officials (NACCHO)
ZAHN, Matthew, MD
Medical Director, Epidemiology
Orange County Health Care Agency
Santa Ana, CA
National Association of County and City Health Officials (NACCHO) (alternate)
DUCHIN, Jeffrey, MD
Health Officer, Public Health – Seattle and King County
Professor in Medicine, Division of Allergy and Infectious Diseases
University of Washington School of Medicine and School of Public Health
Seattle, WA
National Association of Pediatric Nurse Practitioners (NAPNAP)
STINCHFIELD, Patricia A., RN, MS, CPNP
Director
Infectious Disease/Immunology/Infection Control
Children’s Hospitals and Clinics of Minnesota
St. Paul, MN
National Foundation for Infectious Diseases (NFID)
SCHAFFNER, William, MD
Chairman, Department of Preventive Medicine
Vanderbilt University School of Medicine
Nashville, TN
National Foundation for Infectious Diseases (NFID) (alternate)
DALTON, Marla, PE, CAE
Executive Director & CEO
National Foundation for Infectious Diseases (NFID)
Bethesda, MD
National Medical Association (NMA)
WHITLEY-WILLIAMS, Patricia, MD
Professor and Chair
University of Medicine and Dentistry of New Jersey
Robert Wood Johnson Medical School
New Brunswick, NJ
Pediatric Infectious Diseases Society (PIDS)
ANDERSON, Evan, MD
Professor of Pediatrics and Medicine
Pediatric and Adult Infectious Diseases
Children’s Healthcare of Atlanta and Emory University Hospital
Emory University School of Medicine
Atlanta, GA
Pediatric Infectious Diseases Society (PIDS) (alternate)
SAWYER, Mark H, MD
Professor of Clinical Pediatrics
University of California, San Diego School of Medicine
San Diego, CA
Pharmaceutical Research and Manufacturers of America (PhRMA)
ROBERTSON, Corey, MD, MPH
Senior Director, US Medical, Sanofi Pasteur
Swiftwater, PA
Society for Adolescent Health and Medicine (SAHM)
MIDDLEMAN, Amy B., MD, MSEd, MPH
Professor of Pediatrics
Chief, Section of Adolescent Medicine
University of Oklahoma Health Sciences Center
Oklahoma City, OK
Society for Healthcare Epidemiology of America (SHEA)
DREES, Marci, MD, MS
Chief Infection Prevention Officer & Hospital Epidemiologist
ChristianaCare
Wilmington, DE
Associate Professor of Medicine
Sidney Kimmel Medical College at Thomas Jefferson University
Philadelphia, PA
The Advisory Committee on Immunization Practices [ACIP]
All of the following in this section is directly sourced and reproduced from ACIP’s website.
The Advisory Committee on Immunization Practices (ACIP) is a group of medical and public health experts that develops recommendations on how to use vaccines to control diseases in the United States.
ACIP consists of 15 experts who are voting members and are responsible for making vaccine recommendations. The Secretary of the U.S. Department of Health and Human Services (DHHS) selects these members after an application and nomination process. Fourteen of these members have expertise in vaccinology, immunology, pediatrics, internal medicine, nursing, family medicine, virology, public health, infectious diseases, or preventive medicine. One member is a consumer representative who provides perspectives on the social and community aspects of vaccination.
In addition to the voting members, there are 30 non-voting representatives from professional organizations that are highly regarded in the health field. These members comment on ACIP’s recommendations and offer the perspectives of groups that will implement the recommendations. Examples of these professional organizations include:
American Academy of Pediatrics
American Academy of Family Physicians
American College of Nurse Midwives
American College of Obstetricians and Gynecologists
American College of Physicians
The duty of the Advisory Committee on Immunization Practices [ACIP] is to provide advice and guidance to the Director of the CDC regarding use of vaccines and related agents for effective control of vaccine-preventable diseases in the civilian population of the United States.
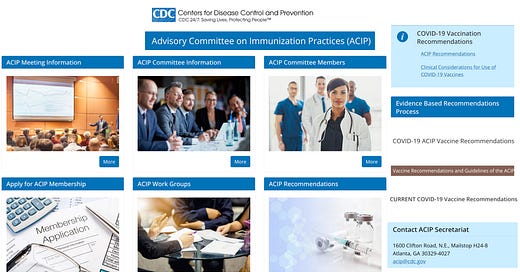
This is the home page for the Advisory Committee on Immunization Practices [ACIP]:
CHARTER, AUTHORITY, LITIGATION VECTORS, EXPOSURE & CULPABILITY
ACIP’s authority is derived from its charter. All of the following is directly sourced and reproduced from ACIP’s charter with aspects relevant to COVID-19 presented in bold font indicating litigation vectors, exposure and culpability relative to statutory authority [also in bold font]:
The ACIP was established under Section 222 of the Public Health Service Act (42 U.S.C. §2l7a), as amended. The Committee is governed by the provisions of the Federal Advisory Committee Act, as amended, 5 U.S.C. App 2).
The ACIP has been given statutory roles under subsections 1928(c)(2)(B)(i) and 1928(e) of the Social Security Act (42 U.S.C. § 1396s(c)(2)(B)(i) and 1396s(e)) and subsection 2713(a)(2) of the Public Health Service Act (42 U.S.C. § 300gg-13(a)(2)).
The Secretary, Department of Health and Human Services (HHS), and by delegation the Director, Centers for Disease Control and Prevention (CDC), are authorized under Section 311 and Section 317 of the Public Health Service Act, [42 U.S.C. §243 and 42 U.S.C. §247b], as amended, to assist states and their political subdivisions in the prevention and control of communicable diseases; to advise the states on matters relating to the preservation and improvement of the public’s health; and to make grants to states and, in consultation with the state health authorities, to agencies and political subdivisions of states to assist in meeting the costs of communicable disease control programs.
The ACIP shall provide advice and guidance to the Director of the CDC regarding use of vaccines and related agents for effective control of vaccine-preventable diseases in the civilian population of the United States. Recommendations made by the ACIP are reviewed by the CDC Director, and if adopted, are published as official CDC/HHS recommendations in the Morbidity and Mortality Weekly Report (MMWR). The CDC Director informs the Secretary, HHS, and the Assistant Secretary for Health, of immunization recommendations. Upon the licensure of any vaccine or any new indication for a vaccine, the Committee shall, as appropriate, consider the use of the vaccine at its next regularly scheduled meeting. If the Committee does not make a recommendation at the Committee’s first regularly scheduled meeting, the Committee shall provide an update on the status of such for the Committee’s review.
The Committee shall provide advice for the control of diseases for which a vaccine is licensed in the U.S. The guidance will address use of vaccines and may include recommendations for administration of immune globulin preparations and/or antimicrobial therapy shown to be effective in controlling a disease for which a vaccine is available. Guidance for use of unlicensed vaccines may be developed if circumstances warrant. For each vaccine, the Committee advises on population groups and/or circumstances in which a vaccine or related agent is recommended. The Committee also provides recommendations on contraindications and precautions for use of the vaccine and related agents and provides information on recognized adverse events. The Committee also may provide recommendations that address the general use of vaccines and immune globulin preparations as a class of biologic agents, use of specific antibody products for prevention of infectious diseases, and special situations or populations that may warrant modification of the routine recommendations.
Committee deliberations on use of vaccines to control disease in the U.S. shall include consideration of disease epidemiology and burden of disease, vaccine safety, vaccine efficacy and effectiveness, the quality of evidence reviewed, economic analyses, and implementation issues. The Committee may revise or withdraw their recommendation(s) regarding a particular vaccine as new information on disease epidemiology, vaccine effectiveness or safety, economic considerations, or other data become available.
In accordance with Section 1928 of the Social Security Act, the ACIP also shall establish and periodically review and, as appropriate, revise the list of vaccines for administration to children and adolescents eligible to receive vaccines through the Vaccines for Children Program, along with schedules regarding the appropriate dose and dosing interval, and contraindications to administration of the pediatric vaccines. The Secretary, and as delegated the CDC Director, shall use the list established by the ACIP for the purpose of the purchase, delivery, and administration of pediatric vaccines in the Vaccines for Children Program.
Further, under provisions of the Affordable Care Act (Section 2713 of the Public Health Service Act, as amended), immunization recommendations of the Committee that have been adopted by the Director of the Centers for Disease Control and Prevention must be covered by applicable health plans.
The ACIP committee is obligated to report-out as follows and as sourced directly from the ACIP charter. The following is directly sourced and reproduced from ACIP’s charter with aspects relevant to COVID-19 presented in bold font indicating litigation vectors, exposure and culpability:
The Committee reports to the Director, CDC. The CDC Director informs the Secretary, HHS and the Assistant Secretary for Health, HHS, of immunization recommendations.
CDC will select a full-time or permanent part-time Federal employee to serve as the Designated Federal Officer (DFO) to attend each committee meeting and ensure that all procedures are within applicable statutory, regulatory, and HHS General Administration Manual directives. The DFO will approve and prepare all meeting policies and agendas, call all of the committee and subcommittee meetings, adjourn any meeting when the DFO deems adjournment to be in the public interest, and chair meetings when directed to do so by the official to whom the committee reports. The DFO or his/her designee shall be present at all meetings of the full committee and subcommittees. In the event that the DFO cannot fulfill the assigned duties of the Committee, one or more full-time or permanent part-time employees will be assigned as DFO and carry out these duties on a temporary basis.
The Committee shall consist of up to 20 Special Government Employees, including the Chair. Members shall be selected from authorities who are knowledgeable in the fields of immunization practices and public health, have expertise in the use of vaccines and other immunobiologic agents in clinical practice or preventive medicine, have expertise with clinical or laboratory vaccine research, or have expertise in assessment of vaccine efficacy and safety. The Committee shall include a person or persons knowledgeable about consumer perspectives and/or social and community aspects of immunization programs. Members shall be deemed Special Government Employees.
According to the U.S. General Services Administration, Special Government Employees are defined as follows:
SGE can be added to an advisory committee in several ways. One is by appointment, one is by contract and one is by use of the Advisory Committee Act. The strengths and weaknesses of the three options are described below.
All SGE must file confidential financial disclosure reports. The legal authority used to hire the SGE will not change that status.
For example, if the SGE is hired under a contract authority, then the SGE still is required to provide independent views to the agency. This is consistent with the Federal Acquisition Regulations which prohibit agencies from entering into employer-employee relationships when entering into contracts for personal services.
SGE could be appointed under an appointment authority. Agencies should be aware, however, that by doing so, the SGE could claim they were entitled to certain benefits stemming from that appointment.
By using a contract authority, it is clear that the SGE is hired to perform under the terms of the contract. However, since the contract is to procure services, it is subject to the Federal Acquisition Regulations.
The following is directly sourced and reproduced from ACIP’s charter with aspects relevant to COVID-19 presented in bold font indicating litigation vectors, exposure and culpability relative to statutory authority [also in bold font]:
The final and best alternative is to neither appoint nor hire the SGE by contract. Rather, the Advisory Committee act expressly contemplates payment to SGEs in section 7. The regulation specifically sets forth a formula at 41 C.F.R. section 101-6.1033. Rather than be concerned about benefit claims on an appointment authority, or about complying with the Federal Acquisition Regulation, the proper authority to pay SGEs is the Federal Advisory Committee Act itself.
The Committee also shall consist of six non-voting ex-officio members from the Health Resources and Services Administration; the Food and Drug Administration; Centers for Medicare and Medicaid Services; National Institutes of Health; Indian Health Service; and the Office of Infectious Disease and HIV/AIDS Policy, HHS; or their designees.
There also shall be non-voting liaison representatives from the American Academy of Family Physicians; American Academy of Pediatrics; American Academy of Physician Associates; American College Health Association; American College of Nurse Midwives; American College of Obstetricians and Gynecologists; American College of Physicians; American Geriatrics Society; America’s Health Insurance Plans; American Immunization Registry Association; American Medical Association; American Nurses Association; American Osteopathic Association; American Pharmacists Association; Association of Immunization Managers; Association for Prevention Teaching and Research; Association of State and Territorial Health Officials; Biotechnology Industry Organization; Council of State and Territorial Epidemiologists; Canadian National Advisory Committee on Immunization; Infectious Diseases Society of America; International Society for Travel Medicine; National Association of County and City Health Officials; National Association of Pediatric Nurse Practitioners; National Foundation for Infectious Diseases; National Medical Association; Pediatric Infectious Diseases Society; Pharmaceutical Research Manufacturers of America; Society for Adolescent Health and Medicine; Society for Healthcare Epidemiology of
America and such other non-voting liaison representatives as the Secretary deems necessary to effectively carry out the functions of the Committee. Liaisons shall be deemed representatives.
Members, including the Chair, shall be selected by the Secretary and shall be invited to serve for overlapping terms of up to four years, except that any member appointed to fill a vacancy for an unexpired term shall be appointed for the remainder of that term. A member may serve 180 days after the expiration of that member’s term if a successor has not taken office.
The records of the committee, established subcommittees, or other subgroups of the committee, shall be managed in accordance with General Records Schedule 6.2, Federal Advisory Committee Records, or other approved agency records disposition schedule. These records shall be available for public inspection and copying, subject to the Freedom of Information Act, 5 U.S.C. §552.
ACIP COVID-19 VACCINE RECOMMENDATIONS
ACIP’s COVID-19 Vaccine Recommendations are captured in the image below and they are sourced from ACIP’s website.
Use of COVID-19 Vaccines in the United States
ACIP’s Use of COVID-19 Vaccines in the Unites States is captured in the image below and it is sourced from ACIP’s website.
ACIP PROTOCOL AND PROCESS
ACIP’s protocol and process for executing is defined in its Role of the Advisory Committee on Immunization Practices in CDC’s Vaccine Recommendations section of its website. The full page is captured below.
The following is directly sourced and reproduced from ACIP’s website with important aspects in bold font:
The ACIP holds three regular meetings each year, in addition to emergency sessions, at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. The purpose is to review scientific data and vote on vaccine recommendations. Meetings are open to the public and available online via live webcast.
The Advisory Committee on Immunization Practices (ACIP) comprises medical and public health experts who develop recommendations on the use of vaccines in the civilian population of the United States. The recommendations stand as public health guidance for safe use of vaccines and related biological products.
During these committee meetings, members review findings and discuss vaccine research and scientific data related to vaccine effectiveness and safety, clinical trial results, and manufacturer’s labeling or package insert information. Outbreaks of vaccine-preventable disease or changes in vaccine supply, such as vaccine shortages, also are reviewed during these meetings. The recommendations include who should receive the vaccine, the number of doses needed, the amount of time between doses, and precautions and contraindications. During ACIP meetings and prior to each voting session, there is designated time for oral public comment, in addition to the opportunity for written public commentexternal icon.
The information that ACIP reviews for each vaccine always includes:
The safety and effectiveness of the vaccine when given at specific ages. Only vaccines licensed or authorized by FDA are recommended, and vaccine manufacturers must conduct rigorous studies to show that a vaccine is safe and effective at specific ages.
The severity of the disease. Vaccines recommended for children and adults prevent diseases that can be serious, potentially causing long-term health problems or death.
The number of people who get the disease if there is no vaccine. Vaccines that do not provide benefit to many people may not be recommended for everyone.
How well a vaccine works for people of different ages. The immune response from a vaccine can vary depending on the age when the vaccine is given.
How practical the recommendations are to put into practice. Factors that can impact the feasibility of implementing a vaccine recommendation can also be considered.
In addition to these meetings, ACIP members participate in work groups. These work groups are active all year to stay up to date on specific vaccines and vaccine safety information. For example, before a vaccine is even licensed by the U.S. Food and Drug Administration (FDA), an ACIP work group will thoroughly review all available scientific information about the vaccine so that they will be prepared to present information to ACIP about the vaccine once it is licensed. At this point, the vaccine already has undergone several phases of testing for safety and efficacy with thousands of volunteers. The licensure process can take several years. The work group carefully reviews data available on the vaccine in order to make recommendations to ACIP, but work groups do not vote on the final recommendation. The work group presents its findings to the entire ACIP at several meetings before ACIP members vote on whether to recommend the vaccine and who should receive the vaccine. Once the CDC Director has approved ACIP recommendations, they are published in CDC’s Morbidity and Mortality Weekly Report (MMWR). Upon publication, the recommendations represent the official CDC recommendations for immunizations in the United States.
Each year, ACIP’s recommendations result in the official U.S. adult and childhood immunization schedules.
The ACIP develops recommendations on how to use vaccines to control disease in the United States.
The Committee’s recommendations are forwarded to CDC’s Director for approval. Once the ACIP recommendations have been reviewed and approved by the CDC Director and the U.S. Department of Health and Human Services, they are published in CDC’s Morbidity and Mortality Weekly Report (MMWR). The MMWR publication represents the final and official CDC recommendations for immunization of the U.S. population.
Professional organizations that work with the ACIP to develop the annual childhood and adult schedules include the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), the American College of Obstetricians and Gynecologists (ACOG), and the American College of Physicians (ACP).
ACIP Recommendations
Complete list of ACIP recommendations published in the MMWR.Immunization Schedules
Links to the childhood, adolescent, catch-up, and adult immunization schedules; plus vaccine recording and screening forms.ACIP Shared Clinical Decision-Making FAQs
Frequently asked questions about ACIP’s recommendations based on shared clinical decision-making
EVIDENCE-BASED RECOMMENDATIONS-GRADE
The following is directly sourced and reproduced from ACIP’s website with important aspects in bold font:
See complete list of GRADE materials linked from MMWR
ABOUT GRADE
CDC vaccine recommendations are developed using an explicit evidence-based method based on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.
Key factors considered in development of recommendations include balance of benefits and harms, type or quality of evidence, values and preferences of the people affected, and health economic analyses.
An Evidence to Recommendation (EtR) framework is used to summarize these key factors
Evidence tables are used to summarize the benefits and harms and the strengths and limitations of the body of evidence.
COVID-19 AS A FACTOR FOR CHANGE
The following notice for healthcare providers is contained on ACIP’s website:
The COVID-19 pandemic is changing rapidly and requires different strategies to maintain clinical preventive services, including immunization. Find up-to-date guidance on childhood, adult, and maternalexternal iconexternal icon vaccination and clinical practice.
ACIP MEMBERSHIP ROSTER
This is the ACIP Membership Roster [image below] sourced on ACIP’s website.
ACIP’s archived membership rosters are also available on its website.
-END OF BRIEF-